Viral immune evasion
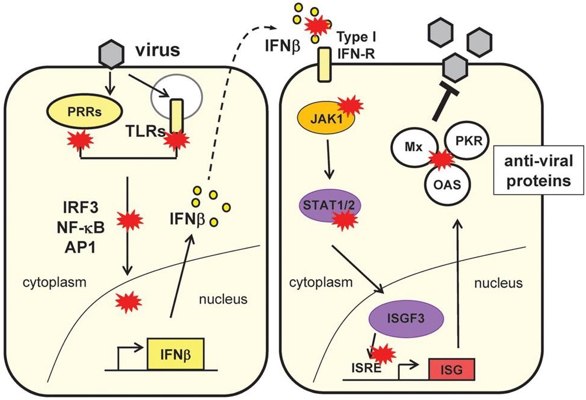
Our interests lay primarily in the study of the strategies employed by large DNA viruses, such as poxviruses and herpesviruses, to escape the host immune response and survive in infected cells as to complete their replicative cycle and establish new infections. Both pox and herpes viruses are enveloped viruses that deliver large (>150 kB) double-stranded DNA genomes coding for multiple proteins. Although fundamental differences exist in their replicative cycles, both families dedicate a great proportion of their vast coding capacity to manipulate the cellular environment and escape the host anti-viral response.
Poxviruses are the only DNA viruses that replicate exclusively in the cell cytoplasm. To be successful and transmit efficiently, poxviruses must have evolved strategies to subvert the arsenal of cytosolic immune sensors present in host cells, including the chief DNA sensor cGAS. cGAS is a DNA-binding enzyme that upon activation synthetises cGAMP, a small second messenger that activates STING and subsequenly the production of interferons and inflammatory mediators. Our work has revealed that virulent poxviruses prevent activation of STING at a step dowmstream of cGAS and that this antagonism is not present in attenuated vaccine strains such as MVA (Georgana et al. 2018, J Virol, PMID 29491158). Our current work involves the characterisation of viral antagonists of this pathway identified by us and others and their impact on the overall immune response and the outcome of infection.
